12. Structure of an iron oxide (Fe2O3) surface as function of temperature and O2 pressure
The structure of the Fe2O3\ (0001) surface as a function of oxygen partial pressure and temperature is computed using first-principles thermodynamics. The results reveal an active four-fold coordinated surface Fe atom which releases oxygen atoms at approximately 850 K at ambient oxygen partial pressure. This property is likely to be related to the catalytic activity of hematite for selective oxidation reactions such as the oxidation from ethylbenzene to styrene.
Keywords: iron oxide, hematite, surfaces, oxygen coverage, first-principles computation, catalysis
12.1. Importance of Iron Oxides
The use of iron and steel as the most common metallic structural material and the ubiquitous presence of oxygen illustrate the technological importance of the interaction of the two elements. Furthermore, iron oxides are used as functional materials including oxidation catalysts, pigments for paints and ceramics, and magnetic materials, e.g. on credit cards. One of the fundamental questions is the structure and reactivity of iron oxide surfaces as a function of temperature and pressure. First-principles calculations provide information and insight of this property at an unprecedented level of detail as shown in this note.
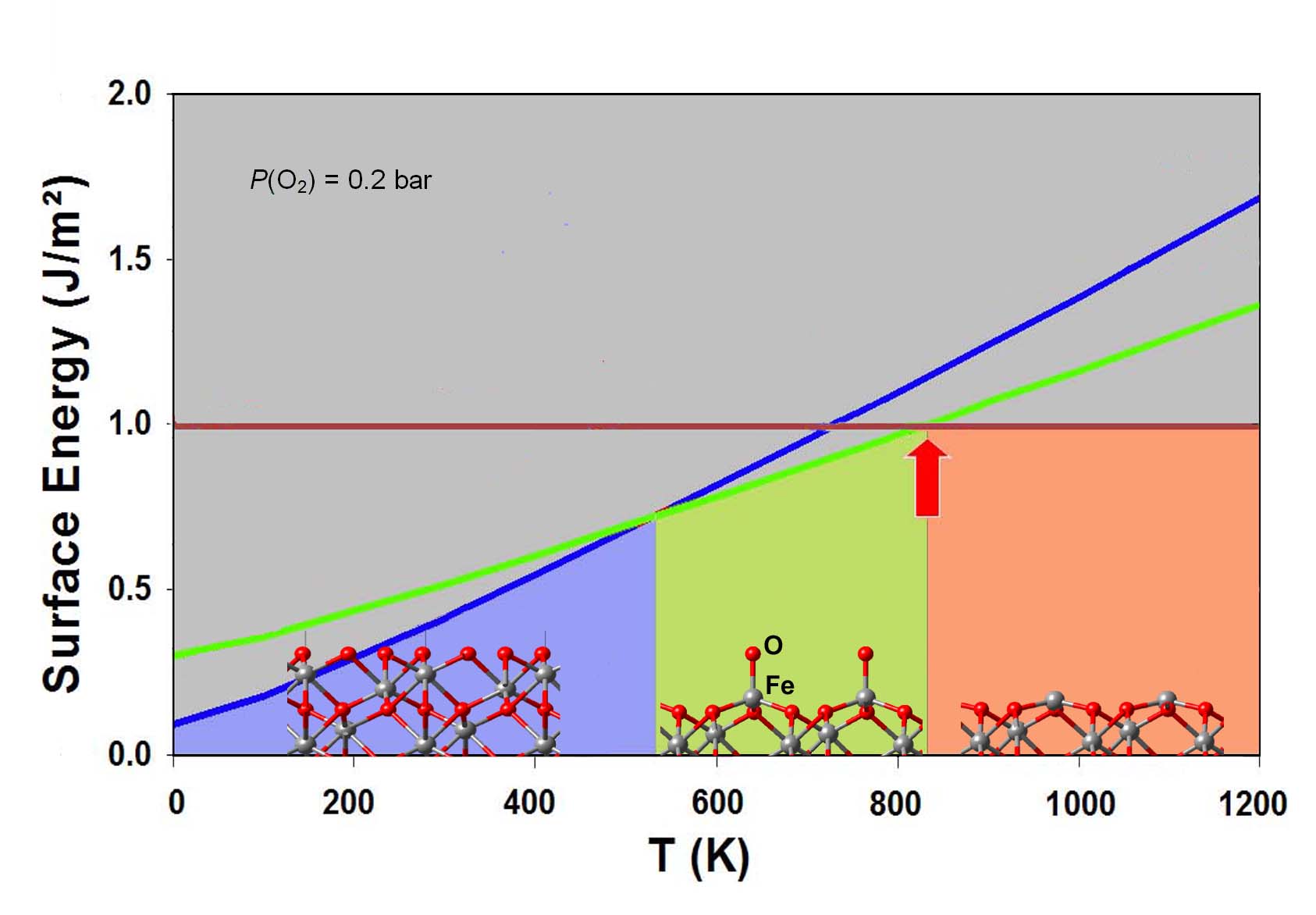
Figure 12.1.1 Computed equilibrium oxygen coverage and structure of an Fe2O3\ (0001) surface as a function of temperature at a constant oxygen partial pressure of 0.2 bar. [1]
12.2. Computed Results
The computed equilibrium structures of a hematite Fe2O3\ (0001) surface is shown in Figure 12.1.1 for a constant oxygen partial pressure of 0.2 bar and a temperature range from 0 to 1200 K.
For temperatures between 0 and 530 K at ambient oxygen partial pressure, the thermodynamically most stable surface of hematite is fully covered with oxygen atoms. In the temperature range between 530 and 850 K, a surface structure becomes thermodynamically more stable. It is characterized by a 4-fold coordinated Fe atom with one oxygen atom bonded perpendicularly to the surface (see the lower middle panel in Figure 12.1.1). These oxygen atoms detach from the surface and form molecular oxygen above 850 K. The remaining surface contains both Fe and O atom.
12.3. Significance
The calculations reveal a four-fold coordinated Fe atom with a reactive oxygen atom, which detaches from the surfaces at approximately 850 K at an O2partial pressure of 0.2 bar. This is close to the operating conditions of industrial dehydrogenation of ethylbenzene to styrene over iron-based catalysts. It is thus likely that the four-fold coordinated ferryl-like iron atom is the active functional group on the iron oxide surface. Computations can now be used to investigate various surface modifications such as replacing Fe by other metal atoms in order to tune the catalytic activity by changing the binding energy of the active oxygen atom.
MedeA modules used in this application
- MedeA Environment including crystal structure builder, surface builder, and analysis tools to assign the required antiferromagnetic structure
- InfoMaticA with structural databases (COD, ICSD, Pearson, or Pauling). For convenience the structure of bulk Fe2O3is retrieved from the databases and used as starting point for constructing surface models.
- VASP
| [1] | W. Bergermayer, H. Schweiger, and E. Wimmer, “Ab initio thermodynamics of oxide surfaces: O2on Fe2O3\ (0001)”, Phys. Rev. B 69, 19540 (2004) (link). |
| download: | pdf |
|---|