13. Adsorption and Dissociation of Iodine Molecules on a Zr Surface
Iodine is a fission product of uranium. It can attack the inner side of zircaloy cladding in nuclear power reactors leading to cracking and fracture. Computations show that iodine molecules adsorb and dissociate on a zirconium surface without an energy barrier. The binding energy of iodine on this surface is large (nearly 300 kJ mol-1 per iodine atom), but the barriers for surface diffusion is only 6.8 kJ mol-1. This gives rise to rapid surface diffusion allowing iodine atoms to reach the crack tips faster than the propagation of cracks.
Keywords: adsorption, dissociation, iodine, zirconium, computation
13.1. Technological context
Iodine has been reported to increase brittle fracture of zirconium with an increase of intergranular cracking [2]. This is a safety concern in nuclear power reactors since this type of fuel-cladding interaction can cause a weakening of zircaloy thus being one of the factors which limit the life time of a fuel assembly.
13.2. Computed results
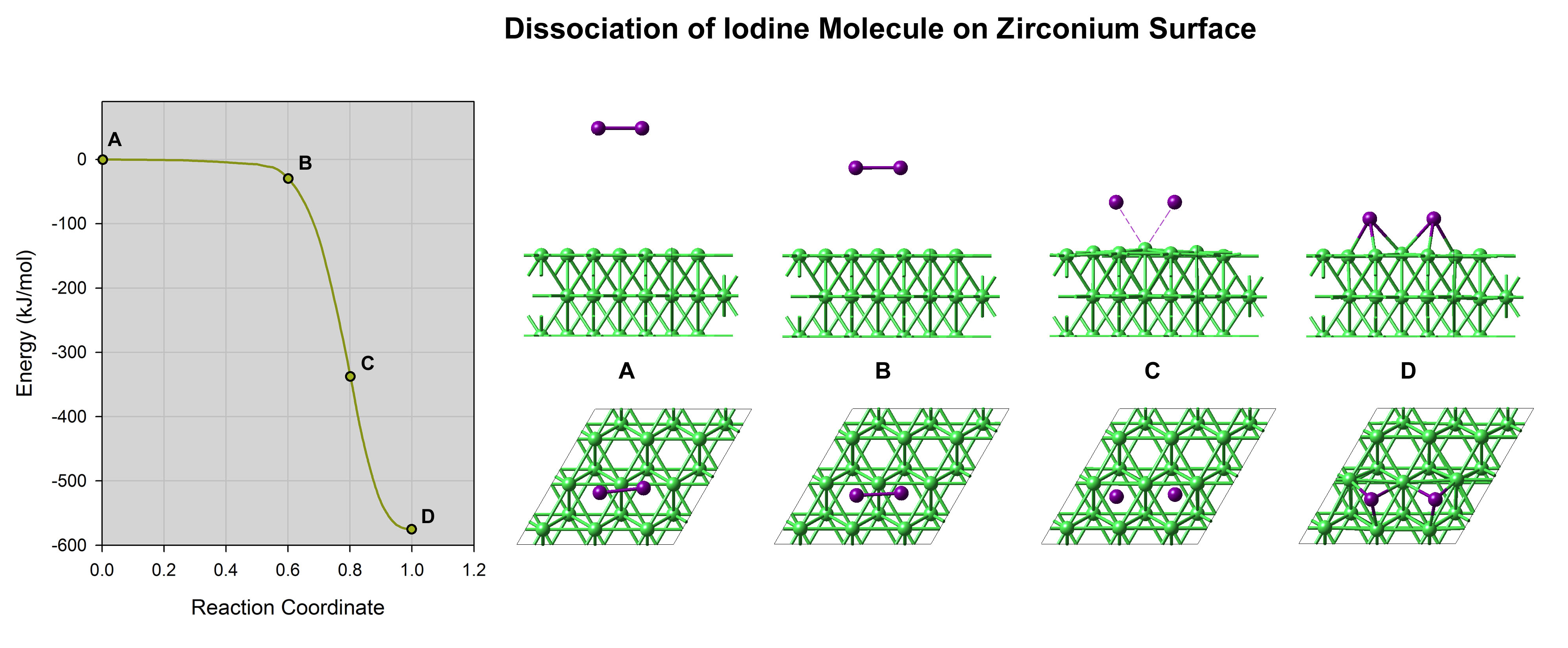
Figure 13.2.1 Computed energy profile of the adsorption and dissociation of an iodine molecule on a Zr(0001) surface. Note the slight elevation of the surface zirconium atoms at point (C), indicating a strong Zr-I affinity.
Computations of the adsorption and dissociation of iodine molecules on the most stable zirconium surface, namely Zr(0001) surface, reveal that this process occurs without any energy barrier as illustrated in Figure 13.2.1. The adsorption energy is large (nearly 300 kJ mol-1 per I atom). The iodine isotherms (Figure 13.2.2), which is obtained by computing the difference in chemical potential between iodine adsorbed on the surface and in the gas phase, shows that a large fraction of the zirconium surface is covered by iodine even at very low partial pressures. Even though iodine binds strongly to the zirconium surface, simulations show that the diffusion barriers are very low, 6.8 kJ mol-1. Hence, iodine can diffuse rapidly on the surface. At a crack surface, the fast diffusion would allow supplying the moving crack tip with iodine enabling an iodine-induced cracking mechanism [3].
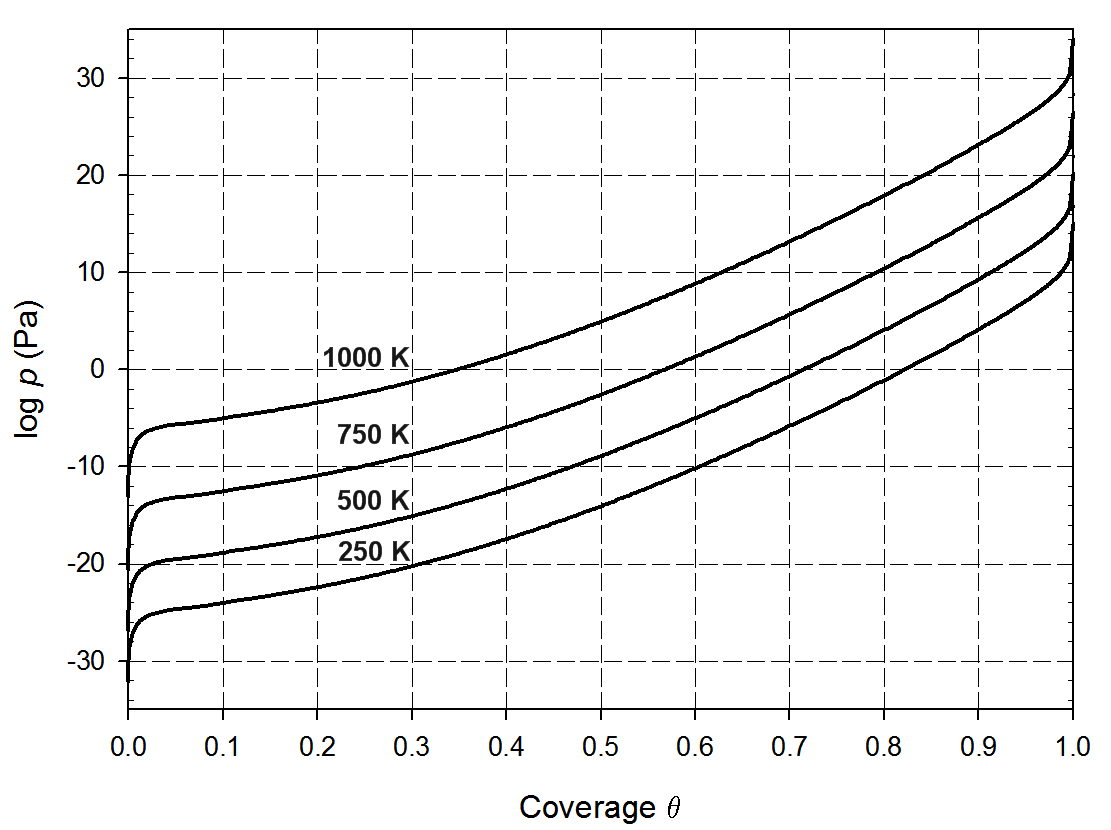
Figure 13.2.2 Computed adsorption isotherms for adsorption of molecular iodine on the zirconium (0001) surface plotted for different temperatures [3].
13.3. Significance
The quantitative understanding and description of interaction of fission products with cladding materials is of fundamental importance for the safe operation of nuclear power reactors. First-principles electronic structure calculations give detailed insight into these interactions and provide quantitative thermodynamic and kinetic data that can be used to model and predict the life-time behavior of fuel rods in nuclear power reactors. This type of simulations can help to increase the safety while potentially allowing a prolongation of the life time of the fuel elements.
MedeA modules used in this application
- MedeA ®[1] Environment framework including crystal structure builder, surface builder, and analysis tools
- MedeA JobServer and TaskServer
- MedeA TSS
- MedeA VASP
- MedeA Phonon
| [1] | MedeA and Materials Design are registered trademarks of Materials Design, Inc. |
| [2] | T. M. Angeliu, B. Kallenburg, D. B. Knorr, and J. D. Ballard, “Development of Fracture Mechanics Method to Evalulate Iodine Stress Corrosion Cracking of Zirconium Alloys”, paper 2164, Proceedings of Top Fuel 2009, Paris, France, September 6-10 (2009) (DOI) |
| [3] | (1, 2) M. Christensen, J. D. Ballard, T. M. Angeliu, J. Vollmer, R. Najafabadi, and E. Wimmer, “Effect of Impurity and Alloying Elements on Zirconium (Zr) Grain Boundary Strength and Iodine Adsorption, Dissociation, and Diffusion from First-Principles Computations”, paper 2165, Proceedings of Top Fuel 2009, Paris, France, September 6-10 (2009) (DOI) |
| download: | pdf |
|---|